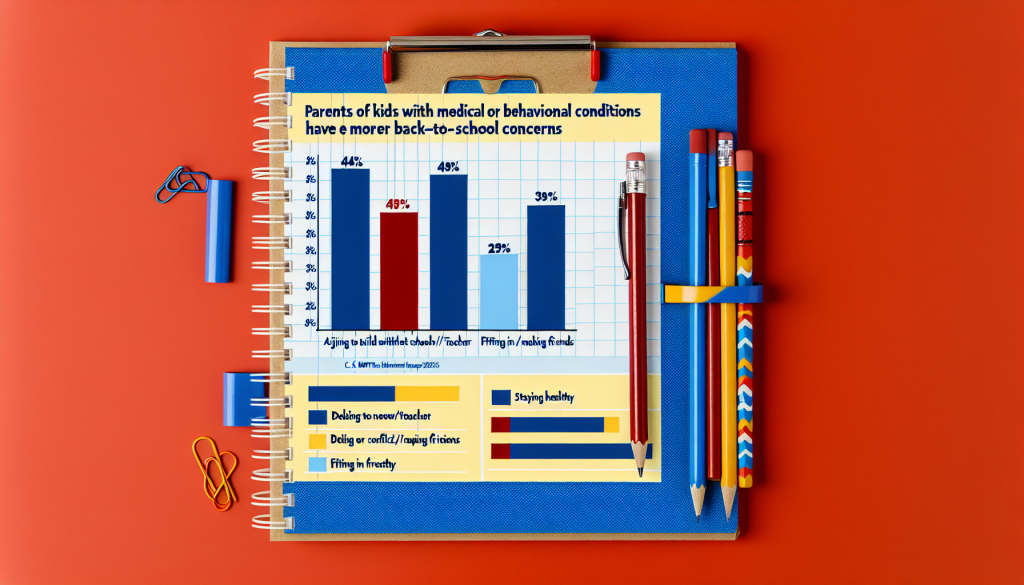
WHO Approves First Two Rapid Antigen Tests for COVID-19
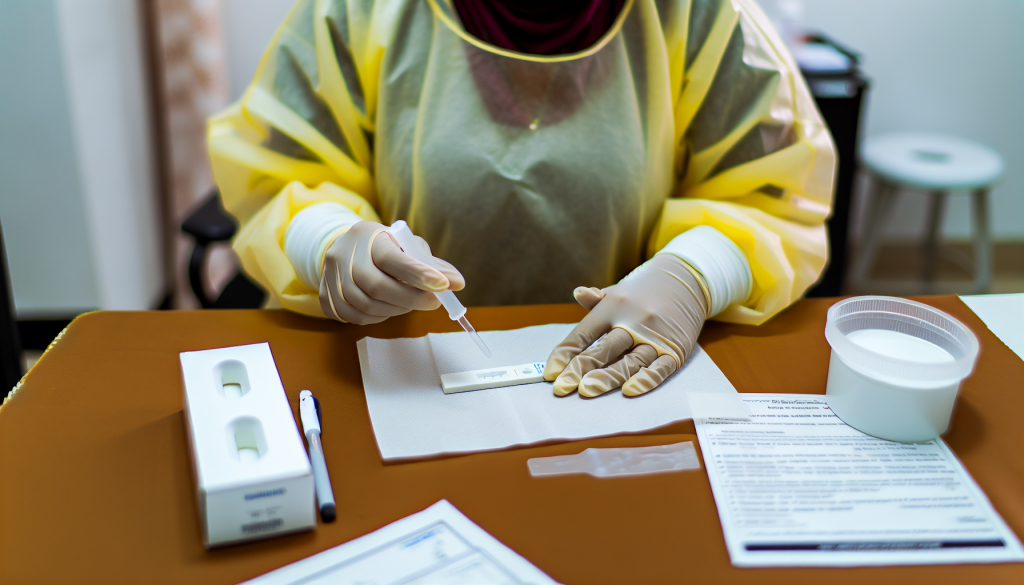
On December 17, 2025, the World Health Organization (WHO) granted prequalification status to two rapid antigen tests designed to detect SARS-CoV-2, the virus responsible for COVID-19. The approved tests are the SD Biosensor STANDARD Q COVID-19 Ag Test and the ACON Biotech Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing). This announcement represents a significant milestone, as it is the first time such tests for COVID-19 have received official prequalification from WHO.
This development follows earlier regulatory steps, where both tests were previously included under WHO’s Emergency Use Listing (EUL). Back in September 2020, the SD Biosensor STANDARD Q test became the first COVID-19 rapid antigen test recognized under the EUL, which supported its widespread adoption in over 100 countries during the pandemic. The EUL serves as a mechanism to speed up access to essential health tools during global health crises by assessing safety and potential benefits when full data sets are not yet available.
Now, with WHO prequalification, these tests are confirmed to meet international standards of quality, safety, and performance for long-term global use. This recognition also makes them available for purchasing by United Nations agencies, global health initiatives, and individual countries. As a result, these tests can be included in pooled procurement initiatives that lower costs and stabilize supply in low- and middle-income countries (LMICs), where access to dependable diagnostic tools is often hindered by financial and logistical challenges.
Although WHO declared an end to the COVID-19 emergency phase over two years ago, the virus remains present globally. While the current circulation of SARS-CoV-2 appears steady, the need for accessible, accurate testing remains high—especially in regions with limited laboratory resources and healthcare infrastructure.
Rapid antigen tests deliver results within 15 to 30 minutes, are cost-effective, and can be administered outside of traditional laboratory settings—such as in community clinics or via mobile healthcare units. This makes them a vital part of the public health response, enabling the fast identification of cases and targeted interventions. These tests serve as an important supplement to molecular tests like PCR, especially in settings with constrained laboratory capacity.
Rapid antigen testing plays a key role in:
• identifying and managing local outbreaks
• safeguarding healthcare staff and high-risk communities
• supporting readiness for future respiratory disease outbreaks
WHO’s overall approach to diagnostics reinforces the need for reliable, decentralized testing solutions as part of efforts to promote universal health coverage and ensure global health security.
Notes to editors
About WHO prequalification and EUL: The WHO Prequalification programme has long supported the availability of safe, effective health products in resource-limited settings. It assesses medical products' quality, safety, and performance, helping global organizations make informed purchases. The programme also provides vital support to national regulatory authorities that may lack sufficient resources. Under its Emergency Use Listing (EUL), WHO carries out expedited reviews of health tools in times of crisis, helping to ensure access to needed products during health emergencies of international concern.