Weekly pill for schizophrenia demonstrates potential in clinical testing
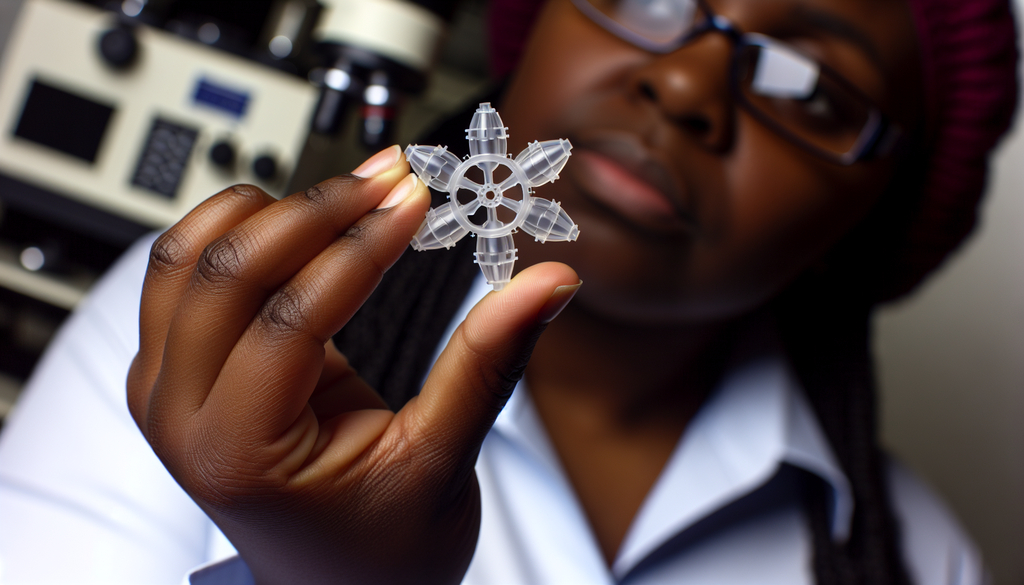
image:
The innovative pill, roughly the size of a standard multivitamin, unfolds into a star-like shape in the stomach, preventing it from passing through the digestive system until all the medication has been dispensed.
Credit: Adam Glanzman
CAMBRIDGE, MA — Many individuals living with conditions such as schizophrenia, various mental health disorders, high blood pressure, or asthma struggle to adhere to daily medication routines. Aiming to solve this problem, scientists at MIT have designed a weekly capsule that gradually releases medication while it stays in the stomach, reducing the burden of daily dosing.
A Phase 3 clinical study led by Lyndra Therapeutics, an MIT-affiliated company, tested this once-weekly pill with a commonly prescribed antipsychotic for schizophrenia. Their findings revealed that the new delivery method maintained steady medication levels in participants, effectively managing their symptoms comparable to traditional daily treatments. The full study is available in The Lancet Psychiatry.
“We’ve managed to transition a daily treatment into a weekly oral dose through a novel technology that could be applied to many other drugs,” said Giovanni Traverso, a mechanical engineering associate professor at MIT, Brigham and Women’s Hospital gastroenterologist, Broad Institute affiliate, and co-author of the study. “Delivering medicine this way ensures consistent dosage and simplifies treatment for patients.”
The concept of this long-acting capsule originated over a decade ago in Traverso’s lab. The device, similar in size to a multivitamin pill, opens into a star formation after being swallowed, allowing it to stay in the stomach until its contents are fully administered.
Senior authors of the research include Richard Scranton, chief medical officer at Lyndra Therapeutics, with Leslie Citrome of New York Medical College as lead author. Other contributors include Nayana Nagaraj, Lyndra’s medical director, and Todd Dumas, senior pharmacometrics director at Certara.
Extended Drug Release
For the past ten years, Traverso's laboratory has created various ingestible devices capable of residing in the gastrointestinal system for extended periods while slowly dispensing medication. In 2016, they introduced the star-shaped delivery system, which has since been refined by Lyndra for clinical testing in patients with schizophrenia.
This device is folded into a capsule format and opens once it reaches the stomach. The six arms extend outward, keeping the device from exiting the stomach via the pylorus too soon. As the arms release the drug steadily over about seven days, they then dissolve or detach, allowing all remaining parts to safely pass through the digestive system.
The drug used in these trials was risperidone, a well-known antipsychotic typically taken daily. Although injectable alternatives exist—from biweekly to bimonthly doses—they require clinical visits and are not always favored by patients.
The research team centered its efforts on schizophrenia, hoping that a simplified oral treatment administered once a week could greatly benefit patients and caregivers alike by supporting treatment adherence.
“In mental health conditions like schizophrenia, where patients may struggle with remembering to take their medication, this delivery method could fill a significant gap,” said Traverso. “Schizophrenia has been a central focus because of those very needs.”
The trial, run by researchers at Lyndra, involved 83 participants across five U.S. locations. Forty-five individuals completed the full five-week program, receiving one risperidone capsule per week.
Analyzing blood samples regularly, the team observed a spike in risperidone levels each time a pill was taken, followed by a gradual decrease—yet the medication consistently remained within the therapeutic range, showing lower variability than daily pills.
Maintaining Therapeutic Stability
Using the Positive and Negative Syndrome Scale (PANSS), the team tracked symptoms throughout the trial and found no significant changes, indicating symptom stability.
“Poor adherence to medication regimens is one of the biggest challenges in managing chronic diseases,” said Citrome. “For schizophrenia patients, inconsistent dosing increases the risk of relapse or hospitalization. A weekly oral option could be crucial for patients who prefer pills over injections.”
Minor side effects like acid reflux or constipation were observed in a few participants early on but resolved quickly. According to the researchers, the promising safety and effectiveness results mark an important step in drug delivery innovation.
“This validates our original hypothesis that a self-contained, long-acting capsule could provide steady therapeutic levels over time,” said Traverso. “Now we can see this actually works in a real-world setting for a significant population of patients with schizophrenia.”
The researchers are planning larger Phase 3 trials before pursuing FDA approval for using this method to deliver risperidone. Preparations are also underway for Phase 1 trials targeting other drugs, including hormonal contraceptives.
“It’s exciting to see a concept born at MIT progressing into advanced clinical trials,” said Robert Langer, MIT’s David H. Koch Institute Professor, a co-founder of Lyndra Therapeutics, and an original contributor to the star capsule concept.
###
This study received funding from Lyndra Therapeutics.
Journal
The Lancet Psychiatry
Subject of Research
People
Article Title
Long-acting oral weekly risperidone (LYN-005) for schizophrenia in the USA (STARLYNG-1): a multicentre, open-label, non-randomised phase 3 trial
Article Publication Date
10-Jun-2025