Researchers reveal novel mechanism of cellular resistance to anticancer treatments
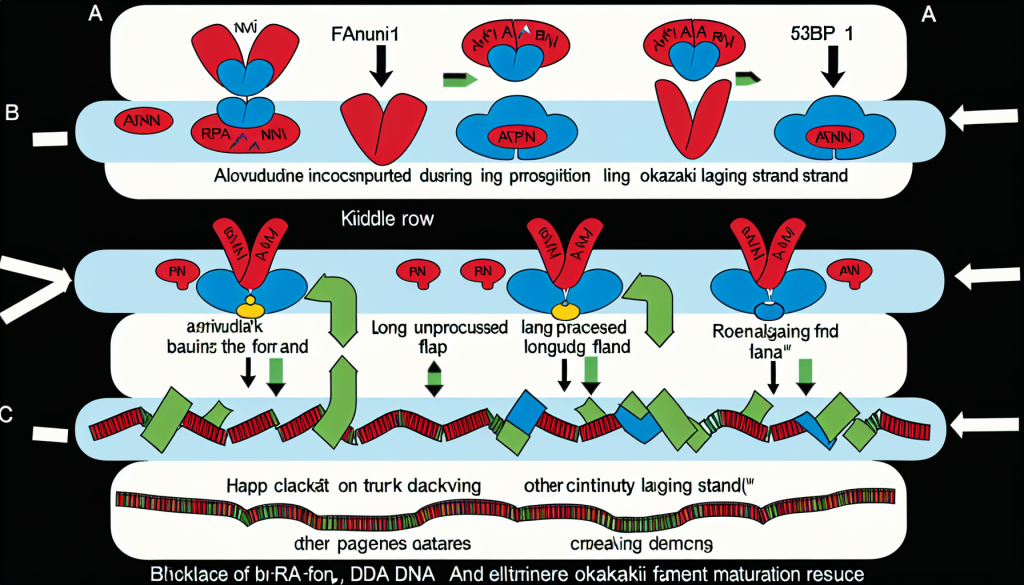
Image:
Without FEN1, the protein 53BP1 builds up, hindering DNA repair. In the absence of 53BP1, other pathways take over to remove long DNA flaps and restore DNA integrity.
Image credit: Tokyo Metropolitan University
Tokyo, Japan – Scientists at Tokyo Metropolitan University have identified a new cellular process that helps protect against the harmful effects of alovudine, a drug used in antiviral and cancer therapies. The research highlights the role of a DNA repair enzyme known as flap endonuclease-1 (Fen1), which helps reduce toxic buildup of the protein 53BP1. This discovery draws attention to the overlooked importance of Fen1, offering new perspectives for cancer treatment and tools for evaluating drug effectiveness.
Chain-terminating nucleoside analogs (CTNAs) mimic the natural building blocks of DNA and have been used since the 1980s to treat viruses and cancer. These compounds incorporate into DNA during replication, especially targeting fast-dividing infected or cancerous cells, thereby halting their growth. However, the way healthy cells manage to resist the toxic effects of these drugs remains unclear, limiting their potential. One such compound, alovudine, was initially promising as an HIV treatment but was discontinued in clinical trials due to safety concerns.
Under the leadership of Professor Kouji Hirota, the research team examined how normal cells defend themselves against CTNA-induced damage. Earlier, the team found that the BRCA1 protein, critical for DNA repair, plays a significant role in protecting cells. Their latest work shifts the focus to Fen1, a lesser-known repair enzyme that trims away short DNA segments—known as flaps—formed during replication.
Using genetically modified chicken DT40 cells, a model frequently used in genetic research, the team discovered that removing Fen1 made cells highly sensitive to alovudine. Replication slowed drastically. Interestingly, when the gene for another protein, 53BP1, was also removed, cells regained their resilience to the drug. Since 53BP1 gathers at damaged DNA sites, this finding suggests that without Fen1, long DNA flaps remain and attract 53BP1. Its presence prevents the removal of these flaps by other mechanisms, blocking DNA replication and causing damage.
The team extended their study to re-examine the function of BRCA1 in this process. Earlier findings showed that BRCA1, part of the homologous recombination (HR) pathway for DNA repair, contributed to cell resistance against alovudine. Loss of either Fen1 or HR led to reduced tolerance, but disabling both caused significantly greater sensitivity. This indicates that Fen1 operates independently of BRCA1, adding another layer of repair capacity within the cell.
This deeper insight into how cells handle CTNA drugs like alovudine could lead to more effective therapies and new methods to detect drug sensitivity, especially in cancers that inherently have lower levels of Fen1. The researchers now aim to expand their study to human cells and explore applications across various cancer types, including solid tumors.
This study received funding from multiple sources, including JSPS KAKENHI Grants-in-Aid (JP25K02256, JP21K19235, JP20H04337, JP22K15040, and 19KK0210), the Tokyo Metropolitan Government Advanced Research Grant (R3-[2]), the Takeda Science Foundation, and the Uehara Memorial Foundation.
Journal
Nucleic Acids Research
DOI
10.1093/nar/gkaf617
Article Title
The flap endonuclease-1 promotes cellular tolerance to a chain-terminating nucleoside analog, alovudine, by counteracting the toxic effect of 53BP1
Article Publication Date
22-Jul-2025
